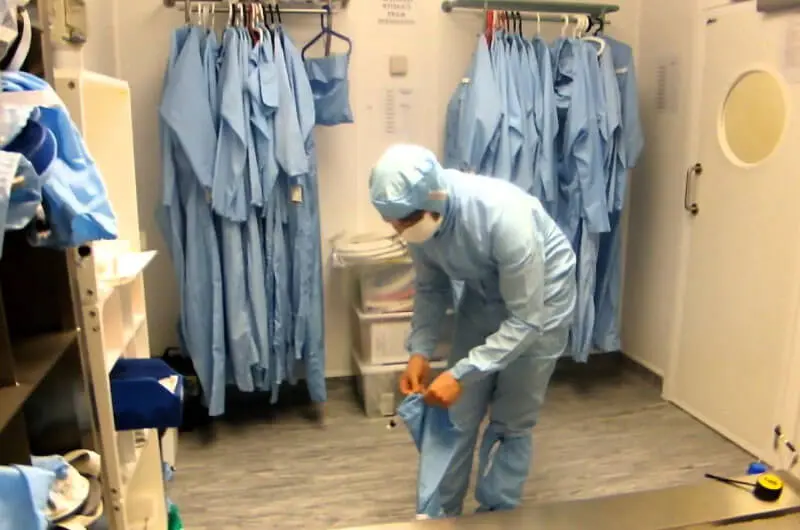
What is Cleanroom?
Cleanroom is a controlled environment where different products are manufactured according to the industry requirement. The concentration of airborne particles in the cleanrooms is controlled to specified limits. The contaminations are usually generated by humans, processes, facilities and equipment and they must be removed from the air.
In the Pharmaceutical production, economical survival of the manufacturer depends on the safety of the finished product. So this makes it crucial for the Pharmaceutical Manufacturer to learn the importance of the Pharmaceutical Cleanroom cleaning and the potential sources of contamination with its effective solution.
The basic function of the Pharmaceutical cleanroom is to protect the manufactured products from contamination. The level of air cleanliness in the Pharmaceutical cleanroom must be regulated by standards like ISO 14644 as this document establishes standard classes of air cleanliness in terms of airborne particulate levels in cleanrooms and clean zones.
What are the sources of Pharmaceutical Cleanroom Contamination?
Being GMP the core motive of any Pharmaceutical Manufacturer, Cleanrooms needs to be really clean. Clean control environment not only prevents the company from contamination but also saves the company from losing its reputation in the Pharmaceutical Industry. What types of Contamination can turn off your Cleanroom essence?
1. 75% of Particulate Contamination happens through Humans- dust, skin, hair, makeup etc. The number of people working in the cleanroom has a direct behaviour on the quantity of air required to dilute and remove the airborne dispersion of contamination from their bodies. The efficiency of the protective cleanroom garments can control the influence of the contamination dispersed by the people. The more effective the cleanroom clothing the less exchange of contamination can ever take place in the air. Protective Cleanroom Garments Dress code like Coverall with hood, Goggles, Gloves for Personal hygiene keeps your manufactured products away from contamination. The temperature level of cleanroom ordinary is 20°C with an RH of 40% ± 5%. Fore moisture sensitive materials, it is required lower RH 25% ± 5%. These temperature levels are dependent on and has an impact of geographical location production and clothing worn. Thus dry bulb temperatures can vary in the range of 18°C to 22°C.
2. 10% of Chemical Contamination spreads through Room structure and Equipment- Oil, grease, Metal ions perfumes etc. Critical cleaning products like Detergents, lubricants and rust & stain remover for stubborn dirt and Wiping and mopping systems enables the Pharmaceutical cleanroom combat against the dangerous contamination.
3.15% of Biological and Radiation Contamination that happens through ventilation are Bacteria, fungi, rodents, ultraviolet light etc. The effective Sterile IPA and Disinfectants works like a magic wand that kills all the microbial contamination in the Pharmaceutical Cleanroom.
What are Cleanroom Standards?
Cleanrooms are generally classified by the cleanliness of air, in terms of safety, reliability, quality and efficiency levels of Cleanroom environment. The history of cleanroom standards dates back in the year 1961, when Technical Manual 00-25-203, the first cleanroom standard was made by the order of American Air Force in USA. In 1963, the first document, Federal Standard 209 of cleanroom facilities regulation was published and entitled as “Clean Room and Work Station Requirements, Controlled Environments”.
The International Organization for Standards (ISO) was published in 1999 that officially replaced Federal Standard 209. This ISO is used in almost all the European and Asian countries to connect all cleanroom requirements as 14644-2 (2000), 14644-4 (2001), 14644- 5 (2004), 14644-7 (2004), 14644- 8 (2006), 14644- 9 (Draft International Standard).
ISO 14644 for Standard for Airborne particulate cleanliness classes in cleanrooms and clean zones.
ISO 14698 for Cleanrooms and associated controlled environments like Bio-contamination control.
What are Pharmaceutical Cleanroom Manufacturing conditions?
1. Sterilized medications manufacturing must be organized in cleanrooms equipped with air chambers for personal access or moving equipment and materials.
2. Original package and product preparation and filling must be done in the detached cleanrooms to minimize risk of product contamination.
The overall cleanliness of the Pharmaceutical Cleanroom is imperative because impurities can cause defects in the products.
What are best Cleanroom practices one can include?
1. A thorough cleanroom gowning requirement and procedures to be developed for avoiding personal contamination.
2. To protect cleanroom and the workers from contamination, ensuring the correct and effective cleanroom supplies in the cleanroom cleaning setting.
3. Training cleanroom personnel on behavioural standards to avoid carelessness and ignorance to cleanroom cleaning.
4. Frequent cleanroom auditing and assessments for the improvements and precautions to major Cleanroom dilemmas.
The object behind developing a contamination-free Pharmaceutical cleanroom is to achieve appropriate microbiological cleanliness levels for the classes of cleanroom for an appropriate period of time. The cleaner the cleanroom environment the safer the Pharmaceutical production. The importance of Pharmaceutical Cleanroom cleaning lies in better health and better living. If you are Pharmaceutical manufacturer and want to attain maximum purity in your Control environment, you can seek Our assistance. We are here to guide you through and through in your Good Manufacturing Practicing.